3.li-un iştirakı il anion polimerlşm
https://limprontastore.it/
studio room for rent in gharafa
Coordinating Anions "to the Rescue" of the Lithium Ion Mobility in .. 2.2 TFSAMs Effect on Viscosity, Ionic Conductivity, and Li + Diffusion. As reported earlier, [] the viscosities of the pure ILs, Pyr 14 DCA, Pyr 14 TFSAM, and Pyr 14 TFSI scale with the size of the anion, that is, with the larger anions leading to higher viscosities, as in ILs the molecular radius of the ions usually affects the shear resistance (and therefore the viscosity) to a much greater . 3.li-un iştirakı il anion polimerlşm. Unraveling anion effect on lithium ion dynamics and interactions in .. In an effort to understand the Li + dynamics in different IL electrolytes, the concentration dependence of the Li + self-diffusion coefficients (D Li) was calculated for four DEME-based IL electrolytes at 393 K [21].In MD simulations, the diffusion of ions was measured by calculating their mean square displacement (MSD), and the slope of the MSD-t curve was then fitted linearly to obtain the .. PDF LIonomers-New Generation of Ionomer: Understanding of Their Interaction .. The PPgMA/IL mixtures (2 and 10 wt%) were blended into a DSM micro extruder at 180 °C for 5 min and injected at 30 °C . Three differ-ent LIonomer types, i.e., depending on the nature of the (cation/anion) pair and of IL con-tent were prepared (Table 1). Table 1 3.li-un iştirakı il anion polimerlşm. PPgMA/IL blends considered in this study. Polymer IL IL Content (wt%) Abbreviation
1956-cı il nəilidir
al sadd room for rent
. Influence of anion structure on ion dynamics in polymer gel .. To overcome the problem of organic solvents, the ionic liquids (ILs), representing salts with a melting point below 100 °C, are widely investigated due to their negligible vapor pressure, nonflammability, high ionic conductivity and the wide electrochemical stability window [3], [4].. Fundamental understanding and practical challenges of anionic 3.li-un iştirakı il anion polimerlşm. - Nature. The structure of layered oxides, such as LiCoO 2 (a), is compared with that of Li-rich layered oxides, such as Li 1.2 Ni 0.13 Mn 0.54 Co 0.13 O 2 (Li-rich NMC) (b) that is derived from Li 2 MnO 3 .. 2.3: Naming Ionic Compounds - Chemistry LibreTexts. The procedure for naming such compounds is outlined in Figure 2.3.1 2.3. 1 and uses the following steps: Figure 2.3.1 2.3. 1 Naming an Ionic Compound 3.li-un iştirakı il anion polimerlşm. Place the ions in their proper order: cation and then anion. Name the cation. Metals that form only one cation.. Molecular-Level Anion and Li - ACS Publications 3.li-un iştirakı il anion polimerlşm. Regulating ion transport is a prevailing strategy to suppress lithium dendrite growth, in which the distribution of ion regulatory sites plays an important role. Here a hyperbranched polyamidoamine (HBPA) grafted polyethylene (PE) composite separator (HBPA-g-PE) is reported. The densely and uniformly distributed positive -NH2 and negative −CHNO- groups efficiently restrict the anion .. Anion Engineering for Stabilizing Li Interstitial Sites in Halide Solid .. Here, we present a new approach, hard-base substitution, and its underlying mechanism to increase the ionic conductivity of halide SEs. The oxygen substitution to Li 2 ZrCl 6 (trigonal, hcp) increased the ionic conductivity from 0.33 to 1.3 mS cm -1 at Li 3.1 ZrCl 4.9 O 1.1 (monoclinic, ccp), while the sulfur and fluorine substitutions were .. 3.4: Ionic Bonding: Anion Formation, Symbolism, and Nomenclature 3.li-un iştirakı il anion polimerlşm. describe its ionization process, determine the charge that will result upon its ionization, provide the ion symbol for the resultant ion, and. provide the ion name for the resultant ion. Answer a. Answer b. 3.4: Ionic Bonding: Anion Formation, Symbolism, and Nomenclature is shared under a not declared license and was authored, remixed, and/or .. Revealing the Anion-Solvent Interaction for Ultralow Temperature .. The 1.5 m LiFTFSI-BFPE electrolyte with strong anion-solvent interaction enables LiNi 0.8 Mn 0.1 Co 0.1 O 2 (NMC811)||Li (20 µm) full cell with stable cyclability even under −40 °C, retaining over 92% of initial capacity (115 mAh g −1, after 100 cycles). The anion-solvent interactions insights allow to rational design the electrolyte .
liqueur de genepi
judo üzrə dünya çempionatı
. Li Coordination of a Novel Asymmetric Anion in Ionic Liquid-in-Li Salt .. We analyze the influence of the asymmetry of the anion on coordination and transport processes in a Li salt/ionic liquid system. The relatively new asymmetric 2,2,2-trifluoromethylsulfonyl- N -cyanamide (TFSAM) anion was investigated in Pyr 14 TFSAM (1- x ) LiTFSAM x over a broad concentration range (up to x = 0.7 Li salt) and was compared to .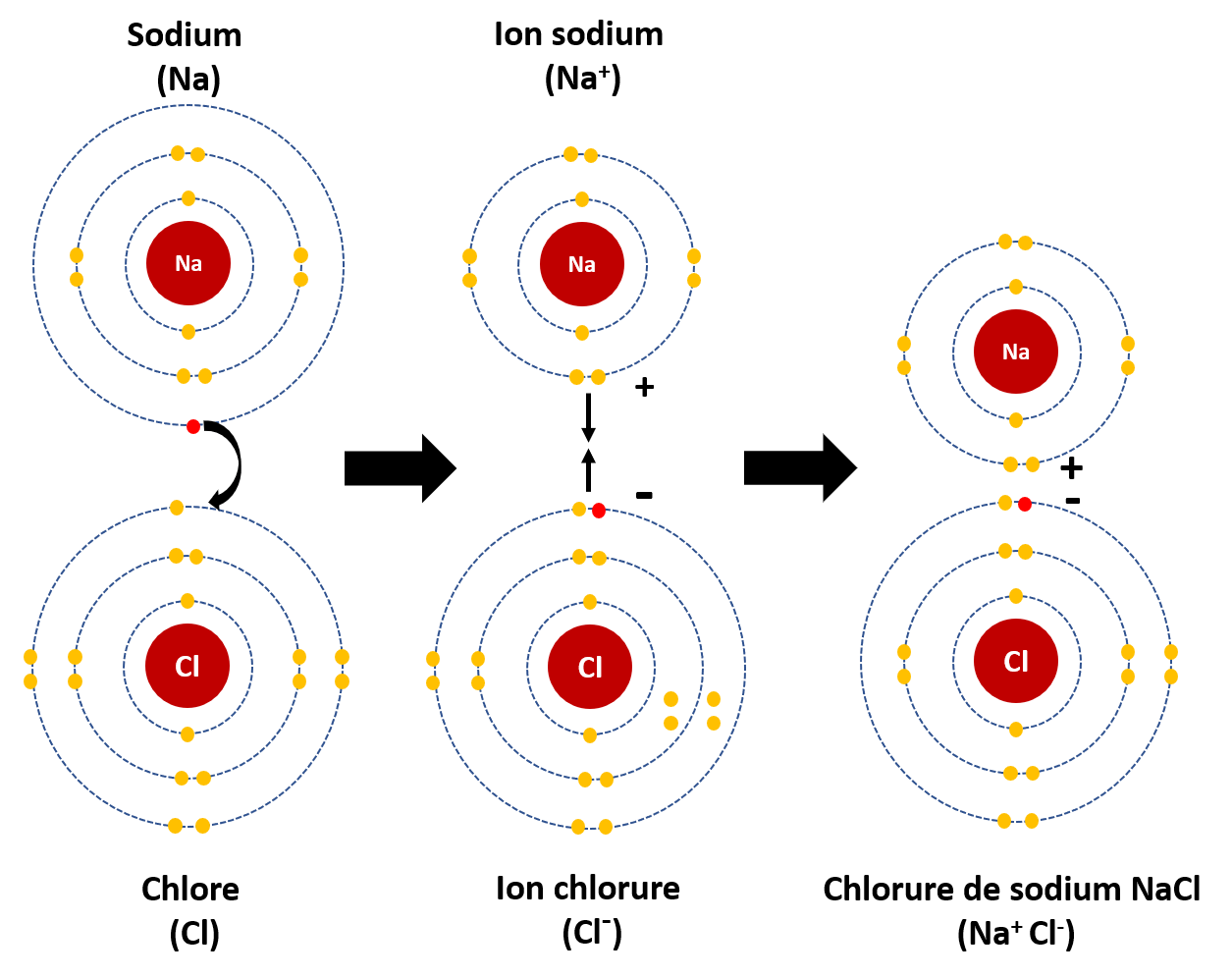
4.3: Ionic Compounds and Formulas - Chemistry LibreTexts. The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. Most cations have charges of [+1] through [+6] while most ions have charges of [-1] through [-3]. Trick: Set # of Anions = Charge of Cation and. set # of Cations = Charge of Anion.
harga polycarbonate per meter
全職法師漫畫免費
. Anion-Dipole Interactions Make the . - Wiley Online Library. Anion-dipole interactions can make homopolymers self-assemble like an amphiphilic block copolymer. Generally, common homopolymers cannot self-assemble into multiple nanostructures. Here, it is reported that anion-dipole interactions can enable a number of homopolymers to achieve a variety of self-assembly behaviors in aqueous solution.Such interactions and self-assembly features have been . 3.li-un iştirakı il anion polimerlşm
土地 と 建物 の 名義 が 違う
barbie öltöztetős jatekok ingyen
. Cation- and Anion-Mediated Supramolecular Assembly of Bismuth and .
tematica pentru majorat
edmark

The use of antimony and bismuth in supramolecular chemistry has been largely overlooked in comparison to the lighter elements of Group 15, and the coordination chemistry of the tripodal ligands [Sb(3-py) 3] and [Bi(3-py) 3] (L) containing the heaviest p-block element bridgehead atoms has been unexplored.We show that these ligands form a common hybrid metal-organic framework (MOF) structure .. Naming ions and ionic compounds (article) | Khan Academy. Lesson 1: Ionic bonds Apply: predicting ion formation Science > High school chemistry > Chemical bonding > Ionic bonds Naming ions and ionic compounds Google Classroom Learn how to name positive ions (cations), negative ions (anions), and ionic compounds involving main group elements.. Poly(Ionic Liquid) as an Anion Exchange Membrane for a 3.3 V Copper .. In summary, we have successfully developed an anion exchange membrane with PDADMATFSI PIL coated on PP as the separator for a 3.3 V Cu-Li battery. The separator is positively charged and can effectively block the migration of Cu ions from cathode to the anode, thereby significantly improving Coulombic efficiency and stability of Cu-Li battery.. 3.3: Anions - Chemistry LibreTexts. In the 1920s, we learned that these conditions could usually be treated easily with the addition of iodide anion to the diet 3.li-un iştirakı il anion polimerlşm. One easy way to increase iodide intake was to add the anion to table salt
дамски боти и ботуши разпродажба
budapesti állások jófogás
. This simple step greatly enhanced health and development. Large amounts of iodide ion are also found in seaweed such as kelp (see picture above .. Ions polyatomiques (leçon) | Khan Academy. Cet article traite des ions polyatomiques 3.li-un iştirakı il anion polimerlşm. Comme le préfixe poly- signifie "plusieurs", un ion polyatomique est un ion qui est composé de plusieurs atomes. Cest ce qui les différencie des ions monoatomiques qui ne contiennent quun seul atome. Parmi les ions monoatomiques, on peut citer, par exemple, Na + , Fe 3 + , Cl − ou bien dautres.. 1-Allyl-3-methylimidazolium-based ionic liquids
το φυλλο τησ γιαγιασ
cfare domethenie ka emri im
. - ScienceDirect. The structure of each IL (Fig. 1 a) was optimized by the (B3LYP) exchange correlation functional and the 6-311 ++G (d, p) basis set implemented in the Gaussian09 suite program [36], [37], [38].The 3D structures of ILs are shown in Fig 3.li-un iştirakı il anion polimerlşm
цена на грам злато
41. heti lottószámok 2015
. 1 b with their stable conformations and their surface charge distributions. The electrostatic potential map (ESP) of polar and nonpolar domains of the IL is . 3.li-un iştirakı il anion polimerlşm. Common Anions Table and Formulas List - ThoughtCo. Updated on February 04, 2019 An anion is an ion that has a negative charge 3.li-un iştirakı il anion polimerlşm. Here is a table listing common anions and their formulas: Table of Common Anions Writing Formulas of Salts Salts are compounds composed of cations bonded to anions
The resulting compound carries a neutral electrical charge. 3.li-un iştirakı il anion polimerlşm. 3.5: Ions and Ionic Compounds - Chemistry LibreTexts. With Al 3 + and O 2−, note that neither charge is a perfect multiple of the other. This means we have to go to a least common multiple, which in this case will be six. To get a total of 6+, we need two Al 3 + ions; to get 6−, we need three O 2− ions. Hence the proper ionic formula is Al 2 O 3. 3.li-un iştirakı il anion polimerlşm. Le Lithium | Superprof. Carte didentité du Lithium
рулетка с шотове
patisen sénégal recrutement
. Le Lithium est un élément chimique métallique, de symbole atomique Li et de numéro atomique Z = 3 3.li-un iştirakı il anion polimerlşm. Sa structure électronique est donc la suivante : (K) 2 (L) 1 soit 3 électrons répartis dans les différentes couches 3.li-un iştirakı il anion polimerlşm. Dans le tableau périodique, il est situé sur la deuxième période et dans la première colonne : il appartient à la famille des alcalins.. 4.3: Formulas for Ionic Compounds - Chemistry LibreTexts 3.li-un iştirakı il anion polimerlşm
Mg2+Cl−Cl− (4.3.2) (4.3.2) Mg 2 + Cl − Cl − 3.li-un iştirakı il anion polimerlşm. Now the positive and negative charges are balanced
河原 の あべ メニュー
. We could write the chemical formula for this ionic compound as MgClCl MgClCl, but the convention is to use a numerical subscript when there is more than one ion of a given type— MgCl2 MgCl 2. 3.li-un iştirakı il anion polimerlşm.







